OverView
Status Update:
2025.09.27
By now some of the below is either out of date or out of scope.
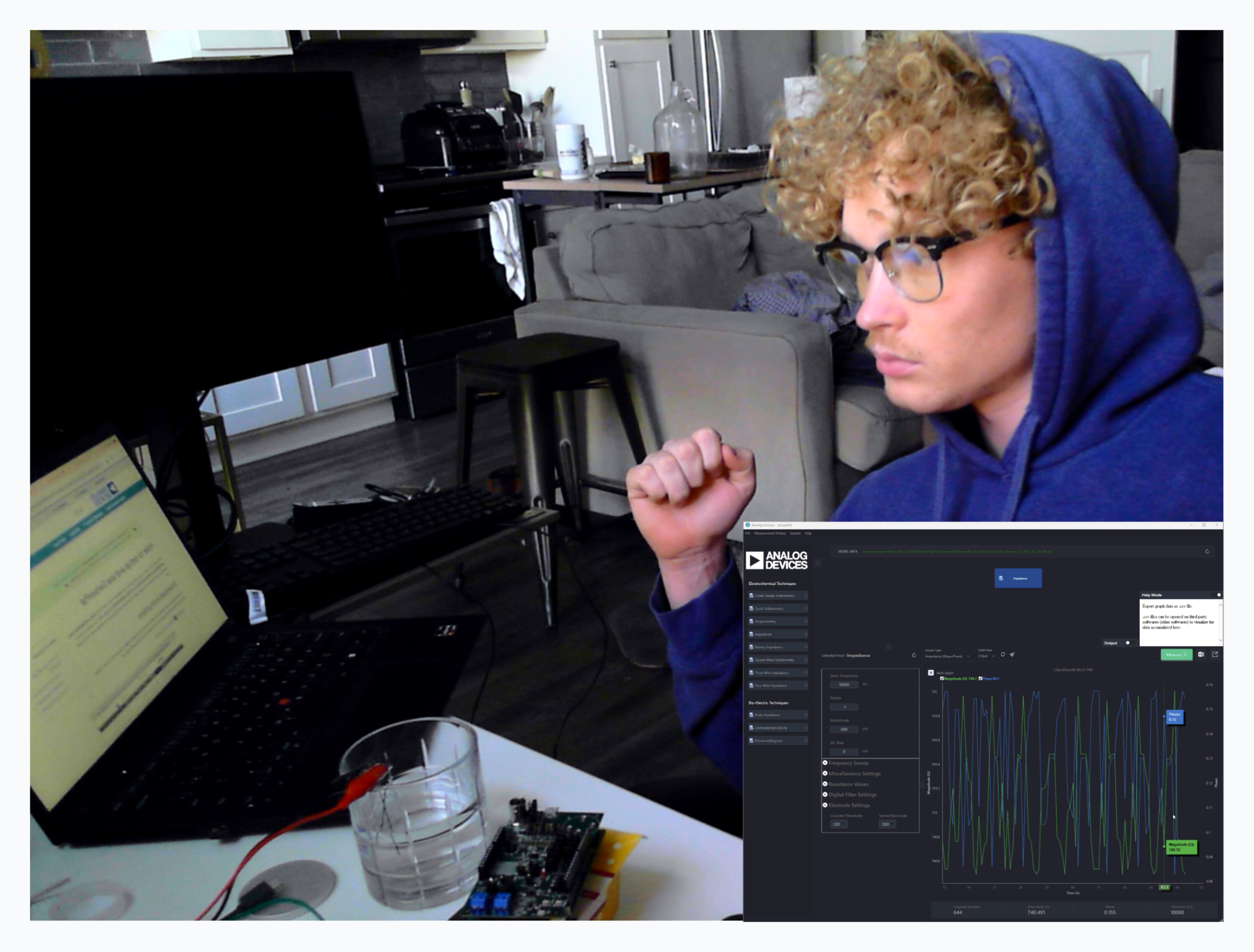
All critical elements have been obtained and we are now working on getting prelimary measuremnets
Aligatory calmped graphite in salted distilled water yielded very good results
Largest block right now is simply costs as we need to get platinum and silver wire (after mistakenly buying 0.03 instead of 0.3mm via ali)
TLDR; Being drunk is fun, being too drunk sucks and not being drunk enough can also suck in certain situations. I have a 4 drink range that I love to be in and want to be able to get there and stay there. Breathalyzers suck bc they can only be used infrequently and require a user to take an explicit action to get a reading. Ideal end state - continuous readings that don't require a user action, data transmission to iPhone via Bluetooth which then displays current BAC, trajectory, speed up/slow down indicator, provides proactive notifications, etc.
Goal: To develop a continuous, wearable, minimally-invasive biosensor capable of monitoring Blood Alcohol Content (BAC) in real-time. The device should require no user interaction for measurements, transmit data to an iPhone via Bluetooth, and provide visualizations and proactive notifications related to current BAC, trend trajectory, and speed of change.
Key Components & Design Breakdown:
1. Sensor Insertion and Physical Design
Needle Requirements:
Originally we were using hollow microneedle idea but pausing due to sourcing difficulty
Length: unknown for now, targeting interstitial fluid in dermal layer)
Gauge: 25G preferred (260–420 µm ID)
Insertion Techniques:
Breakaway needle (ideal, but hard to source)
Slotted insertion tools
Manual or spring-loaded injectors (based on Medtronic-like CGM tech)
Fixation:
- Adhesive patch with sweat- and motion-resistant material
2. Electrochemical Sensing Chemistry
Enzyme Reaction Layer:
Alcohol Oxidase (AOx) from Pichia pastoris (10–40 U/mg)
Immobilized in chitosan (1 wt% in 1% acetic acid)
- highly deacetylated, high-purity chitosan that dissolves readily in dilute acetic acid and forms a viscous solution suitable for biomedical or sensor-related applications (e.g., coating electrodes).
Interference-Rejecting Layer:
Poly(o-phenylenediamine) (PPD) electrodeposited onto electrode surface
Applied from 0.2 M acetate buffer (pH 5.2)
Diffusion-Limiting/Biofouling-Resistant Layer:
Polyvinyl chloride (PVC) dissolved in tetrahydrofuran (THF)
With 1 mM Triton X-100 to adjust alcohol permeability and reduce protein adhesion
Electrode Materials:
Working electrode: Platinum wire/microdisc
Reference electrode: Ag/AgCl
Counter electrode: Optional external wire or planar on-chip
Layered Construction Protocol:
Electrodeposit PPD (interference blocker)
Immobilize AOx in chitosan (1:1, ~10mg/ml AOx)
Crosslink with PEGDE
Add another chitosan layer (1 μl)
Cast PVC/Triton X-100 topcoat (1.5 μl of 2% PVC in THF + Triton)
3. Electronics
Potentiostat Chip:
Analog Devices AD5940 + EVAL-ADICUP3029 board (for prototyping)
chronoamperometry and biasing for enzymatic detection
Production Version:
Custom PCB with AD5940 or equivalent
BLE-enabled microcontroller (e.g. nRF52840 or ESP32 with BLE)
Rechargeable LiPo battery
4. iPhone App Features
Core Display:
Current BAC
Trend line (rise/fall over past 30-60 min)
Forecasted trajectory (extrapolated from current rate of change)
"Sweet spot" visualizer (ideal BAC range, e.g., 0.09–0.17%)
Smart Notifications:
If BAC is rising too fast: "Slow down"
If BAC is falling below threshold: "Have another if you want to stay buzzed"
If BAC is plateauing in optimal range: "You're golden"
Optionally: Suggestions based on time of night, drinks logged, metabolism estimate
Settings & Calibration:
Manual calibration mode (sync with breathalyzer)
Personalization options (weight, sex, drink history)
Data export and sharing for research or personal records
6. Challenges and Next Steps
Challenges:
Positioning of electrode(s)
Long-term stability of AOx enzyme at body temperature
Preventing signal drift over hours/days
Preventing skin reactions or sensor rejection
Biofouling and immune response
Calibration drift with time and metabolism differences
Sensor attachment durability (for >8h sessions)
Sourcing
Ability
Readings from Enzyme via Potentiostat
Getting Sensor Inside Arm
This mf actually busted open the deployment what a g
Needle
Hollow
4mm to 10mm length
21 to 26 gauge, 25 thinner, more common, 21 used for larger sensors (multiple electrodes on one)
A 0.2 mm diameter (200 µm) fits well inside 25G to 27G needles, typically having an inner diameter of 260–420 µm
Lets look for a 6mm (hard keeper) by a 25g
Okay so medtronic is not doing any kinda threading technique in this one,
- But in another they are
Options
Breakaway - probably best option but hard to find
Bend and insert
slotted
Fuck it due redoing the BOM from China
| ID | Title | CAS | Category | Purpose Description | Criticality | Source Status | Price | | | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | | 1 | Alcohol Oxidase (AOx, powder) | 9073-63-6 | 🧪 Enzyme | Oxidizes ethanol → H₂O₂; core biorecognition element for BAC sensing. | ✅ Essential ❗ | ✅ | 240 | | | 2 | Platinum-Iridium Wire (Pt/Ir, 90/10) | – | ⚙️ Electrode | Serves as the Working Electrode (WE) to oxidize H₂O₂. High electrocatalytic activity for amperometry. | ✅ Essential ❗ | ✅ | 40 | | | 3 (can’t find) | Silver Wire (Ag, 0.5 mm Ø) | 7440-22-4 | ⚙️ Electrode | Base material for creating Ag/AgCl reference electrode (RE). | ✅ Essential ❗ | | | | | 4 | Ferric Chloride (FeCl₃) | 7705-08-0 | 🧪 Reference | Chloridizes Ag → AgCl (creates Ag/AgCl RE). | ⚠️ Important ❗ | | 10 | | | 5 | Chitosan (medium-MW, water-soluble) | 9012-76-4 | 🧪 Polymer | Immobilizes AOx on Pt/Ir; forms enzyme matrix. | ✅ Essential ❗ | | 20 | | | 6 | Glacial Acetic Acid also at walmart | 64-19-7 | 🧪 Solvent | Dissolves chitosan to make 1 wt % chitosan solution (pH ≈ 4–5) for enzyme coating. | ✅ Essential ❗ | | 70 | | | 7 | Poly(ethylene glycol) diglycidyl ether (PEGDE) | 39443-66-8 | 🧪 Crosslinker | Crosslinks chitosan/AOx layer, stabilizing enzyme layer. | ⚠️ Important ❗ | Toxic btw | 30 | | | 8 | o-Phenylenediamine (PPD) | 106-50-3 pivoting to CAS 95-54-5 | 🧪 Polymer | Electrodeposited permselective membrane on Pt/Ir to block interferents (ascorbate, urate, acetaminophen). | ✅ Essential ❗ | | 10 | | | 9 | Polyvinyl Chloride (PVC) resin | 9002-86-2 (approx.) | 🧪 Polymer | Forms diffusion-limiting outer film over enzyme; controls ethanol flux and reduces biofouling. | ⚠️ Important 🫶 | | 50 | | | 10 | Triton X-100 | 9036-19-5 | 🧪 Surfactant | Added to PVC solution to impart slight hydrophilicity and tailor ethanol permeability through the PVC film. | ⚠️ Important 🫶 | | 10 | | | 11 | Tetrahydrofuran (THF) | 109-99-9 | 🧪 Solvent | Dissolves PVC + Triton X-100 for casting the final membrane. Requires fume hood or well-ventilated area. | ⚠️ Important 🫶 | toxic btw | 10 | | | 12 | Potassium Dihydrogen Phosphate (KH₂PO₄) | 7778-77-0 | 🧪 Buffer Salt | Along with Na₂HPO₄, makes phosphate buffer pH 7.4 for enzyme stability in artificial ISF. | 🫶 Useful | | 10 | | | 13 | Disodium Hydrogen Phosphate (Na₂HPO₄) | 7558-79-4 | 🧪 Buffer Salt | Along with KH₂PO₄, makes phosphate buffer pH 7.4 for enzyme stability in artificial ISF. | 🫶 Useful | | 5 | | | 14 | Sodium Acetate | 127-09-3 | 🧪 Buffer Salt | With acetic acid, makes 0.2 M acetate buffer (pH 5.2) for PPD electrodeposition. | 🫶 Useful | | 10 | | | 15 (US) | Deionized Water (≥ 18 MΩ·cm) | – | 💧 Solvent | All aqueous steps (dissolving chitosan, preparing buffers, testing). Must be low-ion to avoid noise. | ✅ Essential ❗ | | 15 | | | 16 (US) | Ethanol (≥ 70 %) | 64-17-5 | 🧽 Cleaning | Degreases Pt/Ir and Ag wire before coating; cleans glassware. | ☁️ Soft Support | | 22 | | | 17 (US) | Isopropanol (≥ 70 %) | 67-63-0 | 🧽 Cleaning | Alternative to ethanol for degreasing metal wires and surfaces. | ☁️ Soft Support | | 10 | | | 18 | Potassium Chloride (KCl) | 7447-40-7 | 🧪 Reference | Optionally used with PVB to make a solid-state Ag/AgCl reference (gel). | 🫶 Useful | | 7 | | | 19 | Magnesium Sulfate (MgSO₄) | 7487-88-9 | 🧪 Drying | Drying agent for making anhydrous THF or storing hygroscopic chemicals. | ☁️ Soft Support | | 14 | | | 20 (Revisit) | Heat-shrink Tubing | – | 🛠 Insulation | Insulates Pt/Ir except the tip so coatings only go on the exposed area; prevents shorting. | 🫶 Useful | | 8 | | | | | | | | | | | | | 22 | Magnetic Stir Bar + Stir Plate | – | 🔧 Lab Equipment | Dissolves chitosan in 1 % acetic acid; mixes enzyme–chitosan–PEGDE. | ☁️ Soft Support | | 30 | | | 23 | Pipettes + Microcentrifuge Tubes | – | 🔧 Lab Equipment | Accurately measure small volumes of reagents (e.g., 1–10 µL of enzyme solution, PVC/Triton). | ☁️ Soft Support | | 20 | | | 24 | Small Beakers / Glass Vials | – | 🔧 Lab Equipment | Hold electrodeposition bath (PPD) and artificial ISF during in vitro tests. | ☁️ Soft Support | | 10 | | | 25 | pH Strips or pH Meter | – | 🔧 Lab Equipment | Verify pH of acetate buffer (5.2), phosphate buffer (7.4), and artificial ISF. | ☁️ Soft Support | | 5 | | | 26 | Lab Scale (≥ 0.1 mg resolution) | – | 🔧 Lab Equipment | Weigh small amounts of AOx, chitosan, PEGDE, PPD, PVC powder, Triton. | ☁️ Soft Support | | 108 | | | 27 | Calibration Ethanol Solutions | – | ⚗️ Standards | Known ethanol concentrations (e.g. 0.1 %, 0.2 %, 0.4 % v/v in PBS) for building calibration curve. | ☁️ Soft Support | just get a bottle of everclear | 40 | | | 27.5 | PBS | | | To dillute Ethanol | | | 27 | | | 28 | AD5940 Evaluation Board (EVAL-AD5940ELCZ) | – | 🔌 Electronics | Potentiostat AFE + built-in MCU/BLE. Handles amperometry, filtering, data logging, and wireless streaming to phone. | ✅ Essential ❗ | | | | | 29 | 3D-Printed Electrode Holder/Block | – | 🛠 Hardware | Holds Pt/Ir (WE), Ag/AgCl (RE), Pt (CE) in known geometry; align tips ~3–5 mm apart; provides pogo-pin contact. | ⚠️ Important 🔩 | | | | | 30 | Medical-grade Adhesive Tape (e.g., Tegaderm) | – | 🩹 Attachment | Secures electrode holder to skin during any on-skin test (if choose to do a brief validation). | 🫶 Useful | | | | | 31 (More) | Silver Epoxy or Conductive Epoxy | – | 🛠 Assembly | Joins Ag/Pt wires to connector pins or spring contacts without soldering (Pt/Ir cannot be soldered easily). | ⚠️ Important 🔩 | | 13 | | | 32 | Spring-loaded Contact Pins (Pogo Pins) | – | 🛠 Hardware | Provide a removable interface between coated electrodes (in holder) and the AD5940 eval board’s pads. | ⚠️ Important 🔩 | | | | | 33 (US) | PVC Solvent Masking Tape | – | 🛠 Protection | Protects areas of electrode or holder from stray PVC/Triton spray during casting; peel-off after curing. | ☁️ Soft Support | | | | | 34 (US) | Nitrile Gloves | – | 🧤 Safety | Protect hands from chemicals (THF, PPD, PEGDE, acetic acid) during all prep steps. | ☁️ Soft Support | | | | | 35 (Amazon) | Fume Hood or Well-Ventilated Area | – | 🧤 Safety | Mandatory for handling THF (flammable, harmful vapors) and PPD powders. | ☁️ Soft Support | | | | | 36 (Hard) | Artificial ISF (“in vitro” test solution) | – | ⚗️ Test Medium | Simulated interstitial fluid ( salts, pH 7.4 ) to validate sensor response before any skin test. | ✅ Essential ❗ | | | |
Step-by-Step “How to Go Forward” (Referencing IDs)
1. Prepare Ag/AgCl Reference Electrode (IDs 3, 4, 18, 31)
Cut Silver Wire (ID 3)
- Trim a length (~2–3 cm) of Ag wire (0.5 mm Ø). Polish gently with fine sandpaper to remove surface oxide.
Chloridize with FeCl₃ (ID 4)
Dissolve FeCl₃ in DI Water (ID 15) to ≈ 0.1 M.
Immerse the polished portion of Ag wire for 30–60 s until a gray/white AgCl layer forms. Rinse in DI water.
Insulate with Heat-shrink/PTFE (IDs 20, 21)
- Slide a short segment of heat-shrink tubing (ID 20) or PTFE tubing (ID 21) over the Ag/AgCl so only ~2 mm of AgCl is exposed.
Attach to Pogo Pin/Holder (IDs 29, 32) - This will be hard but we can figure it out
Mount the insulated Ag/AgCl leg into your electrode holder (ID 29).
Use conductive epoxy (ID 31) to bond the wire to its spring-loaded contact in the holder (ID 32). Let epoxy cure.
2. Prepare Counter Electrode (ID 2, 20, 21, 31, 29, 32)
Cut Pt/Ir Wire (ID 2)
- Trim a length (~2–3 cm) of Pt/Ir wire.
Insulate except Tip (IDs 20, 21)
- Slide heat-shrink (ID 20) or PTFE tubing (ID 21) to cover all but ~2 mm at the tip.
Mount in Electrode Holder (IDs 29, 32)
- Insert insulated Pt/Ir CE into holder (ID 29); bond to pogo pin with conductive epoxy (ID 31).
3. Prepare Working Electrode (WE) – Pt/Ir with PPD, AOx, PVC (IDs 2, 8, 1, 5, 6, 7, 14, 9, 11)
Cut & Insulate Pt/Ir (ID 2, 20, 21)
- As above, trim a fresh piece of Pt/Ir, insulate except the tip.
Clean Tip (ID 16 or 17, 15)
- Rinse tip in ethanol (ID 16) or isopropanol (ID 17), then DI water (ID 15). Let dry.
PPD Electrodeposition (ID 8, 14, 15, 28)
Make 0.2 M acetate buffer (pH 5.2): Dissolve Sodium Acetate (ID 14) in DI water (ID 15), add Acetic Acid (ID 6) until pH 5.2.
Dissolve 5 mM PPD (ID 8) in acetate buffer.
Plug Pt/Ir WE, Ag/AgCl RE, Pt CE into EVAL-AD5940ELCZ (ID 28) in 3-electrode mode.
In software, set DAC to +0.65 V vs. Ag/AgCl, hold for 15 min.
Remove WE, rinse in DI water (ID 15), air dry.
Prepare Chitosan–AOx Solution (IDs 5, 6, 1, 7, 15, 22, 23, 26)
Dissolve Chitosan (ID 5): Weigh 1 g chitosan into a glass vial (ID 24), add 100 mL of 1 % acetic acid (ID 6 + ID 15). Heat/mix on stir plate (ID 22) until fully dissolved.
Add AOx powder (ID 1): Weigh AOx to achieve 10 mg/mL final (e.g., 10 mg AOx in 1 mL chitosan solution). Dissolve carefully.
Add PEGDE (ID 7): 1 wt % (e.g., 10 µL PEGDE per 1 mL chitosan/AOx). Stir gently.
Coat WE with Enzyme–Chitosan/PEGDE (IDs 1, 5, 7, 23)
- On PPD-coated Pt/Ir tip, pipette ~1 µL of chitosan/AOx/PEGDE solution (ID 23). Let crosslink ~1–2 h at room temp or 4 °C (chill in fridge if needed).
Add Second Chitosan Layer (ID 5, 23)
- Pipette ~1 µL plain 1 % chitosan (ID 5) over the enzyme spot. Let dry.
Cast PVC/Triton Membrane (IDs 9, 10, 11, 23, 35)
Prepare PVC/Triton solution: In fume hood (ID 35), dissolve 2 g PVC resin (ID 9) in ~98 mL THF (ID 11) using stir bar (ID 22). Once dissolved, add Triton X-100 (ID 10) to 1 mM.
Pipette ~1–1.5 µL of PVC/Triton over the chitosan layer. Let cure in covered container (~4 h at 4 °C).
Mount WE in Holder (IDs 29, 32, 31)
- Insert coated Pt/Ir into holder (ID 29); bond tail end to pogo pin (ID 32) with conductive epoxy (ID 31).
4. Assemble Three-Electrode Array in 3D Holder (IDs 29, 32)
Electrode Spacing
Ensure WE (ID 2), CE (ID 2), RE (ID 3) are mounted ~3–5 mm apart in holder (ID 29) so diffusion zones do not overlap.
Epoxy or glue them into place so tips are co-planar.
Pogo-Pin Interface
- Solder or epoxy the back ends of each wire to a spring pin (ID 32) in the holder so we can press them directly onto the AD5940 eval board pads without soldering.
5. Prepare Buffers & Artificial ISF (IDs 12, 13, 15, 26, 36)
Artificial ISF Base
Can purchase a standard “artificial interstitial fluid” recipe or DIY:
- NaCl 137 mM, KCl 4 mM, CaCl₂ 2 mM, MgSO₄ (ID 19) 1 mM, KHCO₃ 25 mM, Na₂HPO₄ (ID 13) 1 mM, NaH₂PO₄ (ID 12) 1 mM, pH 7.4. Mix in DI water (ID 15).
Keep at 37 °C during tests if possible.
PPD Electrodeposition Buffer (IDs 14, 6, 15)
- Dissolve Sodium Acetate (ID 14) in DI water; add Acetic Acid (ID 6) until pH 5.2.
6. AD5940 Eval Board Setup (ID 28)
Power & Connections
Power via micro-USB. Ensure firmware/drivers (if needed) are installed.
Identify the three pads/pins for WE, CE, RE on the eval board. Confirm which evaluation software (AD5940 GUI or custom script) sets up 3-electrode amperometry.
Configure Amperometry
Set WE potential to +0.60 V vs. RE (Ag/AgCl).
Filter settings: follow recommended (sinc3 OSR=5, sinc2 OSR=1333 → ~38 Hz bandwidth). This is done in the GUI or code.
Sampling interval: start with 5 s or 10 s.
7. In Vitro Calibration & Testing (IDs 15, 27, 28, 36)
Mount Electrode Array
- Press pogo pins (ID 32) onto the eval board pads. Verify good contact.
Baseline in Artificial ISF
- Submerge only the coated tips into stirred artificial ISF (ID 36). Wait ~2 min for baseline current to stabilize.
Ethanol Calibration
Prepare 0.1 %, 0.2 %, 0.4 % (v/v) ethanol in artificial ISF (ID 27).
Add 0.1 % ethanol → record current vs. time (amperometry). Let stabilize. Repeat for 0.2 % and 0.4 %.
Plot current (nA or µA) vs. ethanol concentration → calibration curve.
Stability Test
- In 0.2 % ethanol solution, record continuously for 6–12 h. Check drift ≤ 5 %.
Selectivity Test
- In artificial ISF, add 1 mM ascorbic acid, urate, acetaminophen one at a time → check no significant current change (< 5 % of ethanol signal).
8. (Optional) Brief On-Skin Check (IDs 28, 30, 31, 32)
Only if we want to verify adhesion or gross function on skin Do not need this for an ISF‐only MVP, but it’s included for completeness.
Attach Holder to Skin
- Clean forearm with alcohol (ID 16). Use medical tape (ID 30) to secure electrode holder (ID 29) so tips just touch skin surface.
Connect to Eval Board (ID 28)
- Route a short cable from holder’s pogo pins (ID 32) to eval board. Tape eval board to upper arm or keep in pocket.
Run Amperometry
- Dip only the tips in a droplet of artificial ISF on skin (or use a small wet pad). Verify we get the same baseline (no ethanol). Do a quick 0.2 % ethanol spray onto skin under tips → see response.
Remove & Document
- Peel off tape; inspect skin; verify no irritation (< 5 min).
9. Data Logging & Analysis
Log Raw Data
- Using the AD5940 GUI (or custom Python code), export timestamped current values to CSV.
Convert Current → BAC
- Use calibration curve from Step 7 (Current vs. %EtOH) to convert raw current to estimated %BAC at each timestamp.
Plot & Review
- Plot %BAC vs. time. Check linearity, noise, drift.
10. Cleanup & Storage (IDs 15, 16, 17, 19)
Rinse Electrodes
After tests, rinse all electrode tips (WE, CE, RE) in DI water (ID 15).
Soak briefly in ethanol (ID 16) or isopropanol (ID 17) to remove residual protein/polymers. Air dry.
Store at 4 °C
- Keep coated electrodes in a small sealed container with desiccant (MgSO₄, ID 19) to minimize moisture.
Dispose of Biological Waste
- Discard spent artificial ISF and ethanol solutions per local regulations.
Spreadsheet
https://docs.google.com/spreadsheets/d/1KYuCKvCj9vbvFWmiTJpxvHYkMWBUjb-VHorQu_SflAI/edit?usp=sharing